Abstract
Background: Alemtuzumab (LemtradaTM) is a recombinant humanized anti-CD52 monoclonal antibody that targets T and B lymphocytes, monocytes and eosinophils. It is a Food and Drug administration (FDA) approved drug (approved in November 2014) for relapsing remitting multiple sclerosis (RRMS). Alemtuzumab is also being used occasionally for immune disorders like graft versus host disease (GVHD) and aplastic anemia as well as for B- chronic lymphocytic leukemia (B-CLL) (FDA approved) and sometimes as a part of conditioning regimen for solid and bone marrow transplantations (Campath-1H®). As an interesting paradox to its anti-immune effects, the most serious adverse effects of Alemtuzumab apart from infections are autoimmune effects (AE) (black box warning issued by FDA), affecting various organs mainly thyroid and bone marrow (mainly immune thrombocytopenia). Rare singular cases are emerging of autoimmune hemolytic anemia (AIHA) occurring months after Alemtuzumab infusion; therefore, herein we present the first case series along with review of all the reported cases of AIHA after Alemtuzumab infusion used for treatment of RRMS.
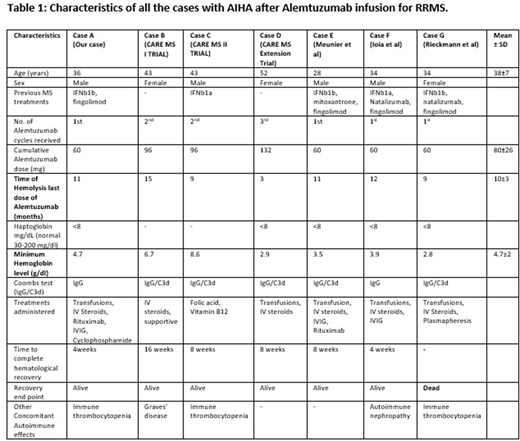
Methods: We did a retrospective chart review of 4 cases of AIHA developing after Alemtuzumab infusion which included 3 cases from the CARE MS-I/II and CARE MS Extension trials (Randomized controlled trials comparing Alemtuzumab versus Interferon beta 1a) (courtesy Sanofi/Genzyme) and 1 case encountered at UT health San Antonio. Simultaneously, we did a literature search using multiple available online databases (PubMed, Ovid, MEDLINE and Cochrane Library) from January 1, 2010 to May 1, 2018 for AIHA associated with Alemtuzumab infusion and found additional 3 reported cases. All of the cases were Direct Antiglobulin test (DAT/Coombs) positive and no other cause of hemolysis was identified. All the analysis was descriptive and exploratory.
Results & Discussion: Out of the total 7 cases reviewed, there were 4 males and 3 females. The common theme amongst all the cases was presence of coombs positive autoimmune intravascular hemolysis occurring 3-15 months (Mean±SD=10±3 months) after the last dose of Alemtuzumab (table 1). In 71% (5/7) of the cases hemoglobin dipped below 5gm/dl requiring multiple transfusions with 1 reported death due to multiorgan failure from complication of severe AIHA. 70% (4/6) cases where steroids were used were not responsive to them requiring the use of some other therapeutic interventions such as Intravenous Immunoglobulin (IVIG), plasmapheresis and second line agents like Rituximab. Interestingly, Alemtuzumab has also been successfully used as a 2nd line agent to treat AIHA especially associated with B-CLL and other immune phenomenon (GVHD, aplastic anemia, conditioning regimen for transplantations) immediate and sustained lymphopenia effect. The proposed mechanism for this "paradoxical" AE related to Alemtuzumab is the reduced diversity and increased regeneration of "self-reactive" T cells during delayed time frame of the "recovery period" of lymphocytes. The reported post marketing incidence of AIHA for Alemtuzumab in RRMS is around 0.13%. Alemtuzumab associated AIHA when used for B-CLL has also been rarely reported.
Conclusion: Severe coombs positive AIHA is a rare life-threatening complication that can occur after Alemtuzumab infusion for RRMS. It can be rapidly progressive, intravascular, severe (hemoglobin frequently reaching to <5gm/dl) and frequently refractory to steroids. It usually occurs between 3-15 months after the Alemtuzumab infusion and can be fatal (with 1 reported death so far). Higher level of awareness is needed by the consulting hematologists of such a rare serious adverse effect that can occur specifically few months after Alemtuzumab infusion and calls for a closer monitoring of patient's hematological status during this recovery period.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal